Characterization research
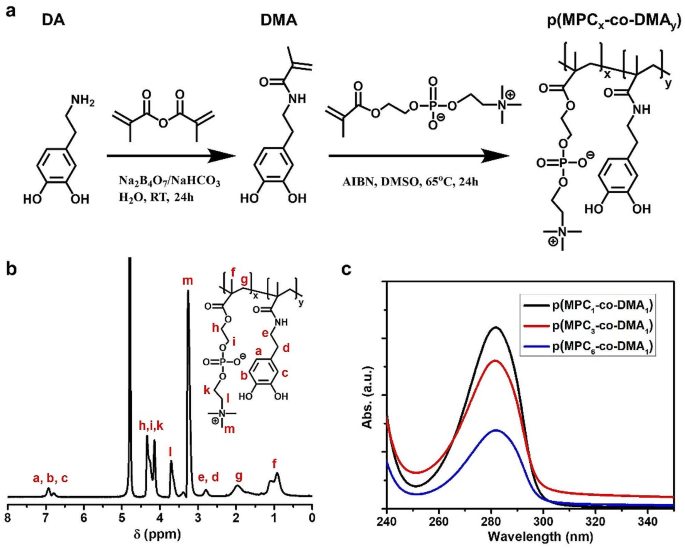
p(MPC-co-DMA) was synthesized and characterised as proven in Fig. 1a. The profitable synthesis of DMA monomer was confirmed as proven in Determine S1. FTIR spectra of DMA monomer depicted the attribute peak at 1650 cm− 1, which signifies the stretching vibration of C = O in amide group (Determine S2). The end result urged the amidation response and polymerizable unit had been conjugated with catechol motif. As well as, the height at 3225 cm− 1 was assigned to -OH stretching vibration of catechol unit. The end result signifies the supply of DMA for adhesive operate. Previous to copolymerization, on condition that MPC monomer has low solubility in DMSO solvent, the profitable p(MPC) synthesis was confirmed utilizing NMR spectra evaluation. As proven in Determine S3, the NMR spectrum of p(MPC) confirmed no proton indicators of residual vinylic double bond at δ = 5.5 − 6.5 ppm, indicating that the resultant powder from our synthesis was in polymer configuration.
A collection of copolymers with various compositions had been produced through the use of the feeding molar ratios of MPC and DMA of 1:1, 3:1, and 6:1. Determine 1b and Determine S4 show the 1H NMR spectra of varied p(MPC-co-DMA) samples utilizing D2O because the solvent. The indicators at 6.79 and 6.93 ppm correspond to three,4,6-trihydrophenyl teams of DMA. As well as, the peaks at 3.26, 3.71, 4.14 and 4.34 ppm are attributed to methylene teams and the methyl teams in MPC. The useful teams of all polymer compositions had been additional analyzed utilizing FTIR spectra. Determine S5 confirmed the attribute bands at 1244, 1170 and 1082 cm− 1, that are attributed to phosphate teams in MPC moiety. Moreover, the absorbance peak at 967 cm− 1 is assigned to the antisymmetric stretches of the C − N bonds within the N-(CH3)3 of MPC moiety. Notably, in varied p(MPC-co-DMA) polymers, a novel peak at 1527 cm− 1 could signify the stretching vibration of C-N bond in amide motif of DMA. To this finish, the NMR and FTIR spectra have confirmed the profitable synthesis of the p(MPC-co-DMA) through free-radical copolymerization. For all the polymer samples, the precise ratios of MPC to DMA are decided by dividing the built-in space of the fragrant protons of DMA (δ = 6.79 to six.93 ppm) by the built-in space of the attribute peaks for MPC (δ = 3.26 ppm). For feeding ratios of MPC to DMA of 1:1, 3:1, and 6:1, the respective ratios for the resultant samples is 2.77:1, 4.49:1 and seven.09:1 (Desk S1). Accordingly, the very best DMA content material is achieved at a price of 26.5 mol% for p(MPC1–co-DMA1). In nature, enriched catechol residues have been documented with values of 30 mol% within the interfacial mussel’s foot proteins (mfp-3 and mfp-5) [41]. Subsequently, p(MPC1–co-DMA1) intently resembles a mussel’s foot protein in time period of catechol content material. The respective catechol content material of p(MPC3–co-DMA1) and p(MPC6–co-DMA1) is eighteen.2 and 12.4 mol%. Determine 1c additional confirms the upper catechol content material within the copolymers at 1 wt% within the order of molar ratio 6:1 < 3:1 < 1:1, which is in step with the molar ratio recorded utilizing 1H NMR. As well as, the catechol content material of copolymers quantified by UV-VIS (Fig. 1c, Determine S6 and Desk S1) can help the information of NMR spectra (Fig. 1b and Desk S1).
The number-average molecular weight (Mn) of the copolymers decided by GPC is proven in Determine S7 and Desk S1. The size of the polymer chain decreases as MPC feeding mass will increase, as proven in Experimental part. It’s troublesome to dissolve DMA homopolymers in natural solvents, and the polymers solely show restricted solubility in dimethylformamide [42]. With out ample safety, radical scavenging capability by catechol hinders the polymerization response as a result of radicals are quenched by semi-quinone radicals, resulting in the formation of intra- or inter-crosslinked construction [43, 44]. Subsequently, the copolymers with larger MPC feeding content material could simply kind an extended polymer chain. Zhang and colleagues additionally discovered that the solubility of copolymer on the molar ratio of 1:3 (ratio between MPC: DMA) was restricted at 4 mg/mL [38]. On this research, our try to extend DMA content material with feeding ratios 1:3 and 1:6 confirmed a sight of catechol oxidation (a darken shade within the polymerization answer) as proven in Determine S8a. Moreover, the vinylic indicators of DMA at δ = 5–6 ppm had been nonetheless seen after the response, indicating the low polymerization efficacy of MPC: DMA feeding ratios at 1:3 and 1:6 (Determine S8b). Subsequently, the very best DMA portion was solely noticed at 1:1 for this research.
Friction measurements
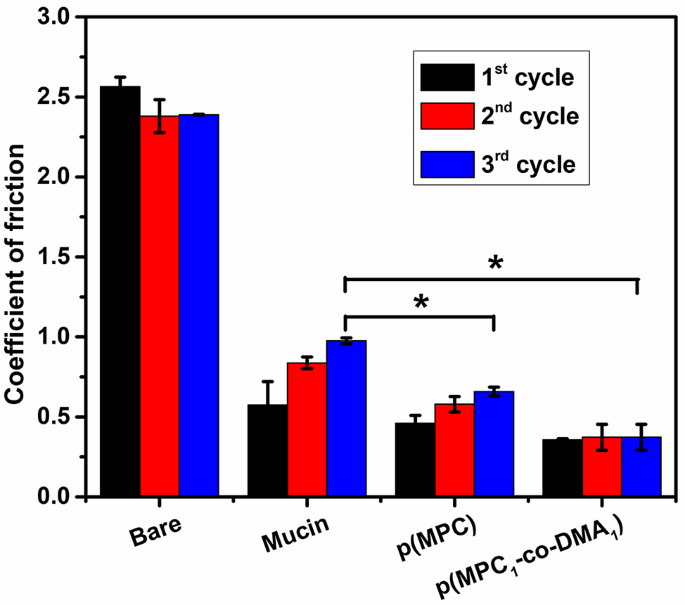
Lubricating brokers can act as an osmoprotectant to ease dry eye discomfort by restoring the physiological osmolarity of tear movie and reducing the impact of hyperosmotic misery to corneal cells [45]. Subsequently, the lubrication properties of mucin and synthesized polymers on silicon tubes had been investigated. For the pristine silicon tube (Naked), the COF was fixed at ∼ 2.5 for all three sliding cycles (Fig. 2). After mucin deposition, the COF worth for the floor initially decreases to ∼ 0.57, after which steadily will increase to ∼ 0.98 after the 2nd and third cycles. The rise in COF tendency was additionally noticed in different polymer coating substrates. As a result of a mucin layer was generated on coating floor through non-specific adsorption, the interfacial shear drive between the coating and the silicon sheet might take away a portion of adsorbed mucin macromolecules after every sliding cycle. Consequently, the steadiness of the lubrication that the mucin-bound floor supplies is compromised. For a polymer-bound floor, the smoothness of the surfaces through which p(MPC) is deposited surfaces will increase to present a COF of 0.46 to 0.66 after three cycles however surfaces on which p(MPC1–co-DMA1) is deposited expertise solely a slight change in COF from 0.36 to 0.37. The distinction in COF values for the 2 polymer compositions most likely displays the performance of DMA. Catechol has a excessive adhesive power on quite a lot of surfaces [41], which might improve the steadiness of the coating in opposition to shear stress. A considerably decrease COF worth for p(MPC)- and p(MPC1–co-DMA1)-coated surfaces than for mucin-bound surfaces on the remaining sliding cycle (p < 0.05) signifies the improved lubrication properties of zwitterionic polymers. Contemplating the excessive dipole second of zwitterionic pendants, the sturdy ionic-driven hydration shell surrounding quaternary amine N+(CH3)3 and phosphonate PO4− shouldn’t be simply squeeze out beneath shear or compression, facilitating a larger diploma of viscous dissipation than for “non-hydration” water [46, 47]. Subsequently, the interfacial friction for surfaces on which MPC is deposited is decrease than the friction for mucin-bound surfaces.
Hydration lubrication analysis of as-prepared polymers. COF of silicon tube (Naked), Mucin-coated, p(MPC)-coated and p(MPC1–co-DMA1)-coated silicon tubes in DI water. The friction assessments had been carried out in every pattern at a traditional load of two N and a sliding pace at 150 mm/mL after three sliding cycles. Values are imply ± SD (n = 3). *p < 0.05 vs. mucin
Mucoadhesive research
Industrial porcine abdomen mucin was used to review the mucoadhesive properties of p(MPC-co-DMA) copolymers. The interplay between catechol-containing copolymer and mucin was first measured utilizing UV-VIS spectroscopy. 100 µL of copolymer options (10 mg/mL) had been added to 1 mL of mucin options (1 mg/mL) to attain a mass ratio of 1:1 in PBS buffer at pH 7.4. As proven in Determine S9a, the mucin answer reveals a robust absorbance sign at 260 nm. There isn’t any vital distinction within the place of the absorbance peak for mucin and mucin-p(MPC). In distinction, UV-VIS demonstrated a slight crimson shift if mucin answer is combined with catechol-functionalized polymer. The absorbance maxima for mucin shifts towards a decrease wavelength area and there is a rise within the catechol content material, which demonstrates {that a} mucin-catechol complicated types [34, 48, 49]. This conduct can be famous by different research, suggesting a change in peptide strands of mucin molecules and their hydrophobicity [48]. As a zwitterionic moiety, MPC segments of the copolymer resist non-specific interplay with biomacromolecules [50]. Therefore, mucin-copolymer complicated formation is solely contributed by DMA segments. Because the catechol diploma will increase, extra reactive websites can be found for conjugation. Moreover, copolymers individually recorded present no signal of catechol oxidation [51, 52], displaying just one absorbance peak centered at 280 nm in PBS buffer (Determine S9a). Therefore, bidentate catecholic hydroxyls can induce sturdy hydrogen bonds [30, 49], permitting preliminary contact for mucin complexation.
Mucins play an important function within the innate safety of the eyes [53]. Therefore, the mucoadhesive properties of p(MPC-co-DMA) had been additional studied utilizing a mucin-deposited substrate previous to in vitro and in vivo research. Mucins had been coated onto a plasma-treated silicon wafer by passive adsorption for 1 h. To confirm the steadiness of the coating, WCA measurement was used to qualify the relative hydrophilicity after mucin deposition. Determine S9b reveals that there’s elevated wetting of the floor of the silicon wafer so there’s a vital discount within the worth of the WCA from 68° to 26.4°. Mucins exhibit a excessive binding affinity with water molecules [54]. This renders coated silicon floor with improved hydrophilicity. After deposition with mucin, copolymers had been allowed to deposit on the substrates for 1 h. Curiously, p(MPC1–co-DMA1) additional induced wetting of mucin movie (WCA at 23.2°), whereas the same end result was not noticed in samples coated with different polymer compositions. As a result of zwitterions can exhibit superior wetting through ionic solvation [15], the lower within the WCA implies the abundance of MPC segments on the mucin layer of p(MPC1–co-DMA1).
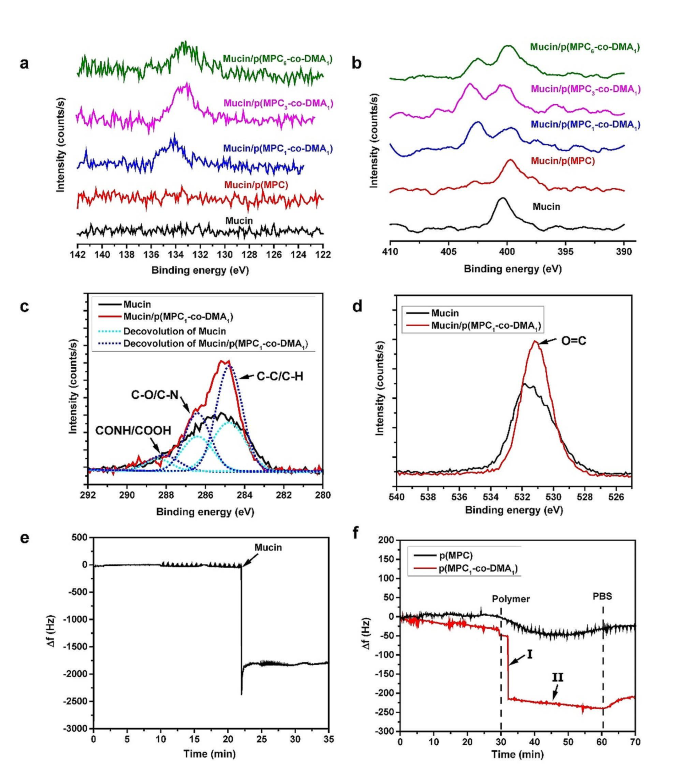
The floor elemental composition of the modified floor was investigated utilizing XPS (Fig. 3). The attribute phosphorus P2p sign at 133.8 eV is simply attributed to the phosphate group of MPC [55]. Determine 3a reveals that the sign is absent for the mucin layer. For various polymer compositions, solely catechol-containing copolymers are immobilized on mucin-coated surfaces. The high-resolution spectra for the phosphorus to carbon ratio (P/C) additionally reveals that there’s a rise from 0.07 to 0.14 (Desk S2), with growing diploma of catechol: p(MPC) < p(MPC6–co-DMA1) < p(MPC3–co-DMA1) < p(MPC1–co-DMA1). The N1s core-level spectra contains a peak for mucin at 400.3 eV, which is related to the first and secondary amines on the N-terminus and peptide bonds in mucins (Fig. 3b) [53, 54]. After the copolymer deposition, a brand new N1s peak at 402.7 eV seems, which demonstrates the existence of the quaternary amine N+(CH3)3 in MPC. The presence of phosphorus and charged N+ are clear proof that zwitterionic moieties are immobilized [58]. Furthermore, introducing acrylamide CH-NH-CH part into DMA will increase N elemental indicators on mucin-deposited floor. Consequently, the atomic ratio of nitrogen to carbon (N/C) is larger for samples on which a copolymer is deposited than for the mucin layer. These outcomes show that DMA options an anchoring performance.
Mucoadhesion analysis of as-prepared polymers. XPS spectra of the modified substrates: Core-level binding power of components: P2p (a), N1s (b), C1s (c), and O1s (d). Adjustments in frequency Δf with time had been recorded with eQCM for mucin deposition (e) and the interplay between polymers on mucin layer (f)
.
Catechols can react with thiol and amine through Schiff base and Michael addition reactions [59]. Thiol-containing residues of mucins have been reported by different research [9, 60]. Nonetheless, the cysteine elements of mucin range between purification batches as a result of variations in animals or purification effectivity [61]. Moreover, cysteine can be not as plentiful as amine residues [34]. Thus, the mucoadhesive properties of polymers are assumed to be most blatant between catechol and amine parts in mucins. To analyze this risk, the core-level spectra of C1s and O1s had been additional analyzed within the mucin layer with and with out deposition of p(MPC1–co-DMA1) (Fig. 3c and d). The C1s spectral sign for mucin layer reveals three respective peak elements for C-C/C-H, C-O/C-N, and CONH/COOH at 284.8, 286.4, and 288.2 eV (Fig. 3c) [56, 62]. Within the p(MPC1–co-DMA1) spectrum, the height intensities for C-C/C-H and C-O/C-N improve, which signifies the presence of the copolymer spine and the tethered residues (acrylamide in DMA and acrylate in MPC) on mucin. Within the O1s spectra, there is a rise in O = C for a binding power of 531.2 eV in p(MPC1–co-DMA1) [63, 64], which reveals that catechol moieties had been oxidized (Fig. 3d). Curiously, catechol moieties had been in a lowered state as proven in UV-VIS spectra, which differs from the end result noticed in XPS. The oxygen-induced conversion of catechol to quinone can happen above pH 5; nevertheless, the method is quite uninteresting, requiring days of monitoring [57]. Therefore, it’s doubtless that catechol-to-quinone conversion didn’t happen after hours beneath a physiological situation throughout UV-VIS measurement. Moreover, amine-rich mucins could speed up the oxidation of catechol residues by selling Michael addition and Schiff base reactions, just like the co-deposition between DA and polyethyleneimine within the work of Xu and colleagues [62].
Actual-time measurement of p(MPC1–co-DMA1) adsorption on the mucin adlayer used eQCM to investigate adhesion conduct. The discount in frequency shift (Δf) is related to adsorbed mass on the sensor. Herein, we first examined the adlayer formation of mucin used on this research. Mucin (0.025 mg/mL) was injected and allowed to adsorb within the circulation cell for 35 min, forming a mucin adlayer with a secure mass density, as proven in Fig. 3e. The adlayer was then rinsed with PBS for 30 min to take away loosely-bound mucin. The polymer compositions had been then allowed to circulation for 30 min earlier than rinsing with PBS as proven in Fig. 3f. For p(MPC) homopolymer, there was a gradual lower in Δf after injection, then a small improve and halt at a mass density of 21.6 ng/cm2. The primary driving drive for non-specific adsorption of p(MPC) is Van der Waal forces, that are frequent however comparatively weak secondary bonds in shut proximity [65]. After p(MPC1–co-DMA1), there was an abrupt lower (section I) adopted by a gradual lower (section II) in Δf. In section I, the copolymer passively diffuses and is absorbs onto mucin adlayer, and the interplay between catechol and mucin supplies anchoring websites to permit secure adhesion of the copolymer. In Section II, the catechol teams that stay on the adsorbed copolymers additionally permit inter-chain interactions with free-floating chains, resulting in a rise within the adsorbed mass density. Consequently, the ultimate mass load for the copolymer is 213.2 ng/cm2, which is roughly 10 instances the worth for p(MPC). To sum up, the adhesive mechanisms for catechol-functionalized polymers have three elements: (1) catecholic functionalization promotes the immobilization of zwitterions on the mucin floor, as proven by the XPS spectra; (2) bidentate catecholic residues of the copolymer permit sturdy and instantaneous hydrogen bonding with the mucin layer; and (3) the catechol-amine adducts that steadily kind between the mucin and the copolymer improve the mucoadhesive properties of the copolymers.
In vitro biocompatibility assessments
Previous to the antioxidant and anti inflammatory analysis, the cytotoxicity of p(MPC) and p(MPC-co-DMA) is decided. The biocompatibility of all artificial polymers is decided utilizing a MTS assay and reside/useless staining. For this work, SIRC cells from the cornea of regular rabbit was used. The mitochondrial dehydrogenase exercise (MTS exercise) for the Ctrl group was outlined as 100%. The metabolic actions and viability ranges of SIRC cells after publicity to polymer samples had been depicted in Determine S10 and S11. After two days of incubation, all of the polymer samples didn’t alter the cell morphology no matter their focus (starting from 0.1 to 1 mg/mL) (Determine S10a and b). Quantitative knowledge for MTS exercise and reside/useless assay additionally supported knowledge from the microscopic photos in that the values for metabolic exercise and cell viability are 95% of the values for the Ctrl group (Determine S10 and S11). Along with cell cytotoxicity, an alkaline comet assay was performed to research the potential genotoxicity of the polymers. Determine S12 indicated intact nuclei with easy edges in all samples. Furthermore, the tail lengths of comet in all compositions confirmed no obvious distinction with the Ctrl (0.9 ∼ 1.2 μm). The end result reveals that no DNA breakage was brought on by the polymers [66, 67]. General, the artificial polymers used on this research exhibited no dangerous impact on SIRC cells. Subsequently, the in vitro and in vivo research had been performed and evaluated utilizing the very best polymer focus of 1 mg/mL.
Antioxidant and anti inflammatory exercise research
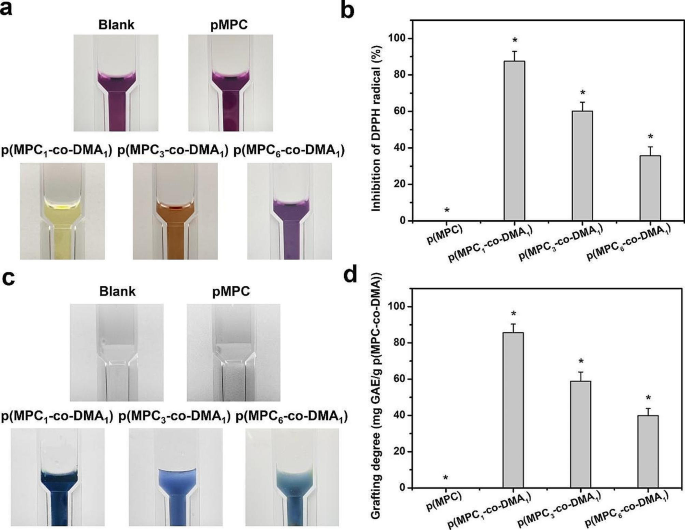
A rise within the quantity of ROS is a significant factor of the DED etiology [68]. The over-expression of intracellular ROS on the ocular floor has varied antagonistic outcomes, together with DNA, lipids, protein harm and cell apoptosis [69]. To find out the antioxidant exercise of the polymers, this research makes use of DPPH to find out the free radical scavenging capability for varied p(MPC-co-DMA) teams. The outcomes are proven in Fig. 4a and b. DPPH antioxidant answer has a most absorbance at 517 nm [70]. As the unconventional scavenging functionality of antioxidant agent will increase, the colour of DPPH answer steadily modified from darkish purple to mild yellow [71]. Quantitatively, the respective radical inhibition percentages of p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) was 87.52 ± 5.36%, 60.14 ± 4.89% and 35.63 ± 4.94%. In distinction, the worth of p(MPC) was just like clean pattern. These outcomes highlighted that the antioxidant functionality was intently related to the diploma of catechol teams inside copolymers, which has obtained vast recognition in different literature [38, 44]. To help this assumption, we continued to conduct Folin-Ciocalteu assay to find out the polyphenol content material of p(MPC-co-DMA). The colour of a p(MPC1–co-DMA1) answer turned darker blue as proven in Fig. 4c, implying a better stage of polyphenols [72]. The polyphenol content material at 85.7 ± 4.7 mg, 59.0 ± 5.0 mg, and 39.9 ± 3.9 mg for gallic acid equal (GAE)/g polymer decreases within the order (Fig. 4d): p(MPC1–co-DMA1) > p(MPC3–co-DMA1) > p(MPC6–co-DMA1).
Anti-oxidation analysis of as-prepared polymers. Images of the response of DPPH reagent (a) and Folin-Ciocalteu reagent (c) with the check samples together with p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1) and p(MPC6–co-DMA1). DPPH scavenging exercise (b) and whole phenolics content material (d) of the assorted samples had been analyzed by UV-VIS spectrophotometry. Values are imply ± SD (n = 5). *p < 0.05 vs. all teams. The clean group: with out p(MPC-co-DMA) pattern
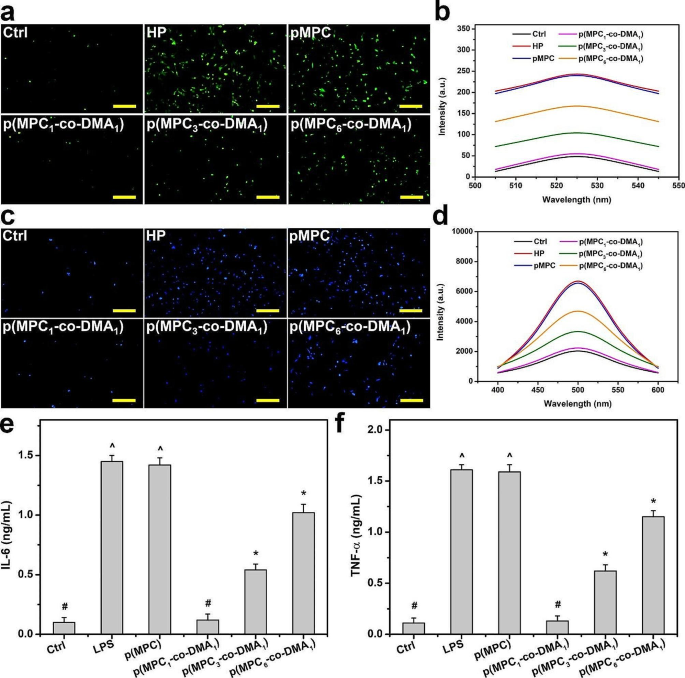
Mobile fashions of oxidative stress had been adopted to evaluate the antioxidant capabilities of p(MPC-co-DMA) supplies in vitro. DCFH-DA, which is a cell-permeable non-fluorescent probe, may be hydrolyzed to grow to be non-permeable DCFH inside cells. DCFH may be additional oxidized by elevated intracellular ROS (superoxide O2−), forming seen fluorescent indicators as proven for HP group (Fig. 5a). The fluorescent indicators from cells lower considerably after two days of incubation with catechol-containing polymers. The larger the focus of catechols in p(MPC1–co-DMA1), the extra the ROS stage reduces to the worth for the Ctrl group (Fig. 5b). Catechol can quench free radicals by supplying hydrogen atoms from the phenolic hydroxyl residues, however catechol can even scale back different reactive brokers by way of electron switch, and the ensuing phenoxyl radical can additional work together with the second radical to kind secure quinone buildings [73]. The regulation of calcium has an important function in chemical signaling, thus the over-expression of intracellular calcium is taken into account a contributing issue resulting in cell loss of life [74, 75]. Subsequently, additional research on intracellular calcium ranges had been performed to confirm cell survivability (Fig. 5c and d). The intracellular calcium stage after H2O2 therapy decreased considerably when cells had been co-incubated with p(MPC-co-DMA) copolymers within the order: Ctrl < p(MPC1–co-DMA1) < p(MPC3–co-DMA1) < p(MPC6–co-DMA1) < p(MPC) = HP. These outcomes affirm that catechol-functionalized polymers inhibit ROS harm.
In vitro anti-oxidation and anti-inflammation of as-prepared polymers. (a) Consultant fluorescent photos and (b) intracellular ranges of ROS measured by the fluorescence depth of DCFH-DA of the SIRC cells after incubation with completely different samples for twenty-four h and additional publicity to H2O2 for twenty-four h. (c) Consultant fluorescent photos of the SIRC cells and (d) intracellular ranges of calcium measured by the fluorescence depth of Fura-2, AM after incubation with completely different samples for twenty-four h and additional publicity to H2O2 for twenty-four h. Ranges of (e) IL-6 and (f) TNF-α from the p(MPC-co-DMA) samples (p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1) and p(MPC6–co-DMA1)). Scale bars: 100 μm. Values are imply ± SD (n = 5). *p < 0.05 vs. all teams; #p < 0.05 vs. LPS, p(MPC), p(MPC3–co-DMA1) and p(MPC6–co-DMA1) teams; ^p < 0.05 vs. Ctrl, p(MPC1–co-DMA1), p(MPC3–co-DMA1) and p(MPC6–co-DMA1) teams
Irritation is an underlying issue for DED [69]. Therefore, the pro-inflammatory components (IL-6 and TNF-α) had been additional examined. Lipopolysaccharide-induced cells had been additionally used as a illness management group (LPS) to imitate DED signs. As depicted in Fig. 5e and f, the expression of IL-6 and TNF-α decreases considerably (p < 0.05) for LPS teams which are topic to p(MPC-co-DMA) therapy. Furthermore, p(MPC1–co-DMA1)-treated group reveals the very best anti-inflammatory functionality of the catechol-containing teams, which demonstrates their antioxidant capability.
Corneal retention research
On this research, the traditional rabbit corneas had been monitored beneath a slit lamp for h12 and 4 days post-instillation to validate any corneal damage brought on by polymers. The rabbit eyes receiving topically instilled polymeric drops confirmed no indicators of corneal redness, edema, or irritation (Determine S13). Quantitatively, all observations of the modified Draize check resulted in scores of zero as proven in Desk S3. Moreover, intraocular strain (IOP) worth after polymer instillment remained comparable with Ctrl group, which confirmed the security of the artificial polymers (Determine S14).
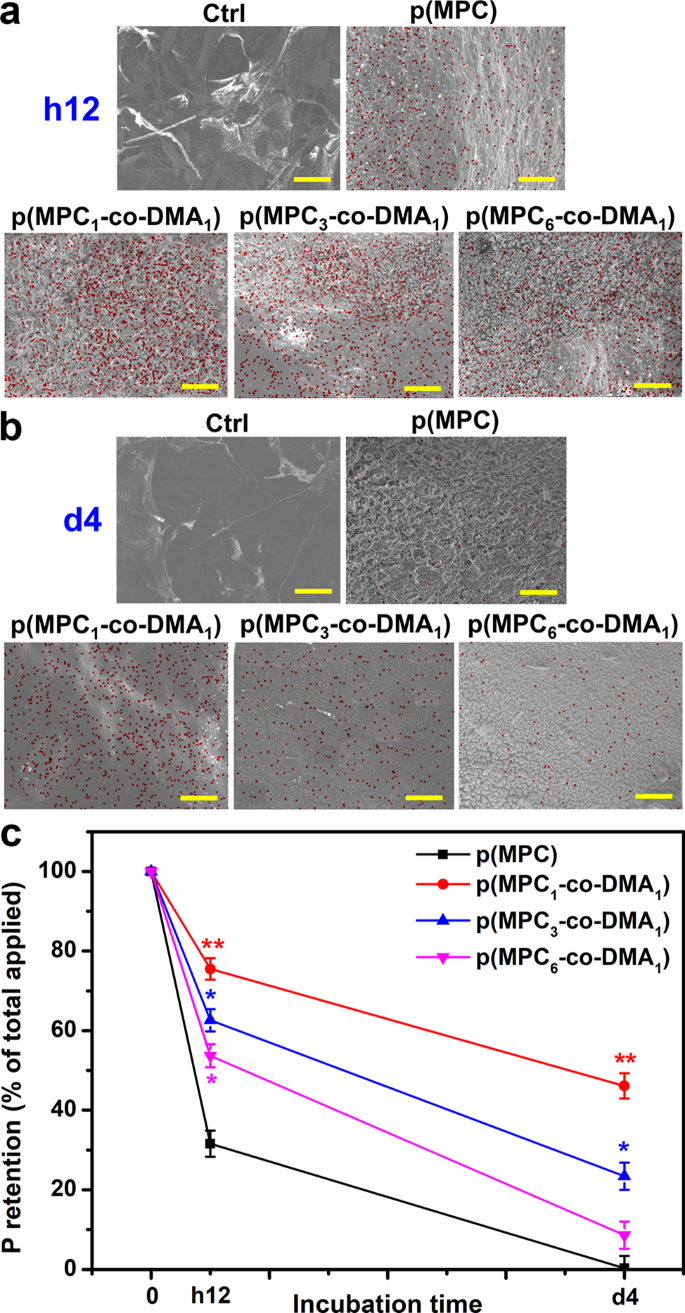
The bio-adhesive properties of p(MPC-co-DMA) copolymers had been additional evaluated in vivo. On this investigation, the content material of p(MPC-co-DMA) retained on the cornea was examined by SEM-EDS after h12 and 4 days of topical administration of nanotherapeutic formulations (Fig. 6a). No elemental phosphorous (crimson dot) was detected within the Ctrl group. The meibomian lipid layer is constituted by the structural group of polar lipids (of which phospholipids are crucial) [76]. Nonetheless, the precorneal tear movie shouldn’t be a component from corneal tissue. This end result could clarify the absence of phosphorus sign on ocular floor. Therefore, the measurement for elemental phosphorous can solely be attributed to phosphorylcholine pendants from MPC-containing polymers. For p(MPC) group, the phosphorus portion was ∼ 31% after h12 of therapy. In distinction, at the very least 50% of the entire phosphorus sign, which corresponds to p(MPC-co-DMA), remained seen on the ocular floor (75%, 62%, and 53% for the feeding ratios of 1:1, 3:1 and 6:1, respectively). The outcomes urged the focus of catechol considerably have an effect on retention effectivity. Remarkably, phosphorus protection of p(MPC1–co-DMA1) remained at about 46%, however the phosphorus protection of p(MPC3–co-DMA1) and p(MPC6–co-DMA1) decreased considerably (23% and eight%, respectively), as proven in Fig. 6b. As demonstrated within the mucin mannequin, the preliminary attachment of the copolymers was as a result of presence of bidentate hydroxyls in lowered catechol. Nonetheless, covalent solidification of amine-catechol adducts is important for long-lasting mucoadhesion [34]. The plentiful catechol residues inside p(MPC1–co-DMA1) probaly promote an inter-chain interplay through phenol-phenol coupling [33], resulting in heightened P retention share.
In vivo mucoadhesion evaluation of nanoformulations. SEM photos of wholesome eyes at (a) h12 and (b) 4 days post-instillation of various formulations. EDS mapping of phosphorus distribution for the corresponding wholesome eyes. Scale bars: 10 μm. (c) Corneal retention of P was analyzed by ICP-OES with the assorted p(MPC-co-DMA) samples. Values are imply ± SD (n = 6). *p < 0.05 vs. p(MPC) group; **p < 0.005 vs. p(MPC) group
Therapeutic efficacy research
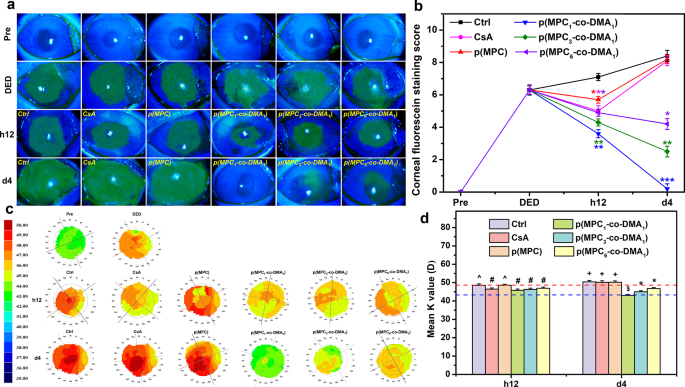
The therapeutic impact of the artificial polymer was investigated in BAC-induced DED mannequin. BAC, which is a preservative utilized in many ophthalmic topical options, can speed up tear movie breakage and evaporation [77]. Rabbits with dry eyes exhibited corneal/conjunctival damage and inadequate tear secretion on the finish of the induction section, just like these of DED sufferers. An examination of the ocular floor and tear movie in a medical ophthalmology examination can point out the development of dry eye illnesses [78]. Medical standards for DED, together with a fluorescein very important staining rating of ≥ 3 and a Schirmer check worth of ≤ 5 mm, had been used to validate the restoration of rabbit eyes after therapy utilizing completely different polymer compositions. For the untreated group (Ctrl group), the DED rabbits that receiving ATS alone exhibited the best fluorescent depth for the experiment. An immunomodulatory agent (CsA) was used as a reference for comparability with the polymers. CsA can alleviate the signs of dry eye and the underlying inflammatory pathologic state [77]. Nonetheless, CsA possesses low solubility and bioavailability [79], limiting its potential therapeutic impact for DED therapy. The CsA group solely confirmed a discount within the space of corneal fluorescein staining after h12 of administration (Fig. 7a). The short-term therapeutic motion was additionally noticed in homopolymer p(MPC). As proven in Fig. 7b, p(MPC) exhibited a discount (p < 0.05) in fluorescein staining rating after h12 of administration. The instillation of hydrophilic p(MPC) can replenish moisture on the ocular floor and stabilize the osmolarity of tear movie [25], however didn’t adhere nicely and didn’t produce an anti-oxidative motion to inhibit the development of DED. Therefore, with out ample bioavailability and bio-adhesive mechanism, CsA and p(MPC) teams have related scores to these for the untreated DED mannequin (Ctrl) at 4 days postoperatively. In distinction, the constructive fluorescent indicators for p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) decreased linearly after h12 and 4 days postoperatively, signifying a gradual mitigation of corneal epithelial harm. Moreover, there was a correlation between catechol focus and the therapeutic impact of p(MPC-co-DMA). With the very best synergistic antioxidant/anti-inflammatory impact demonstrated in vitro, the fluorescein very important staining rating in p(MPC1–co-DMA1)-treated teams lowered to ∼ 0 (p < 0.001), suggesting probably the most pronounced therapeutic impact.
In vivo therapeutic results of nanoformulations. (a) Corneal fluorescein staining photos and (b) fluorescent staining scores of rabbit eyes at preoperation (Pre), after dry eye (DED) induction, and people with experimentally induced DED after topical administration of various formulations for h12 and 4 days. Dry eye animals receiving ATS with out polymer and drug had been management teams (Ctrl). *p < 0.05 vs. Ctrl group; **p < 0.005 vs. Ctrl group; ***p < 0.001 vs. Ctrl group. (c) Corneal topographic values and (d) imply Ok worth from corneal topography of rabbit eyes after h12 and 4 days of topical instillation of various formulations. The blue and crimson sprint traces signify the Pre and DED group values, respectively. Values are imply ± SD (n = 6). *p < 0.05 vs. all teams; +p < 0.05 vs. Pre, DED, CsA, p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams; #p < 0.05 vs. Pre, Ctrl, and p(MPC) teams; ^p < 0.05 vs. Pre, DED, p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams; $p < 0.05 vs. Pre, Ctrl, CsA, p(MPC), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams
In ophthalmology, corneal imaging is used to detect and deal with varied ocular diseases. As well as, corneal topography can be utilized to observe adjustments in response to ocular illness development. As proven in Fig. 7c and d, the inexperienced sign in Pre group is nearly equally distributed, indicating a minimal corneal abnormality. Quite the opposite, DED and Ctrl teams displayed a heterogeneous distribution of inexperienced, yellow, orange, and shiny crimson indicators because of irritation, suggesting a horizontal wavefront aberration. A comparability of the outcomes for all of the teams reveals that the corneal topological map and curvature values for the p(MPC1–co-DMA1) group most have a resemblance to these for the Pre group and show the best therapeutic impact for DED.
DED-associated irritation can speed up corneal endothelial cell loss and alter mobile morphology [80]. Therefore, specular microscopic photos and the endothelial cell density of rabbit eyes in response to topical instillation of the polymers had been investigated at h12 and 4 days postoperatively. Within the Pre group, wholesome endothelial cells on Descemet’s membrane displayed a attribute hexagonal form and had been intently packed collectively (Determine S15a). Quite the opposite, morphological abnormality of endothelial cells was detected within the DED mannequin. The change in mobile morphology grew to become extra evident in Ctrl and p(MPC) teams, together with a big discount (p < 0.05) in endothelial cell density in contrast with DED group (Determine S15a and b). For different teams, as a result of CsA and p(MPC-co-DMA) nanoformulations are bioactive (antioxidant/anti-inflammation), the publicity of DED-induced eyes to the polymers can inhibit the continual deterioration of endothelial cell density after h12 of instillation. Nonetheless, inadequate doses and the shortage of retention mechanisms could hamper the bioavailability of CsA [12, 13], which limits its long-term therapeutic impact as depicted in Determine S15b. p(MPC-co-DMA) copolymers with their superior mucoadhesion and antioxidation can additional stop corneal endothelial harm at 4 days postoperatively. Our findings indicated that amongst all experimental teams, p(MPC1–co-DMA1) not solely therapeutically promoted epithelial regeneration, but additionally effectively preserved cell morphology and density of corneal endothelium throughout 4 days of medical remark.
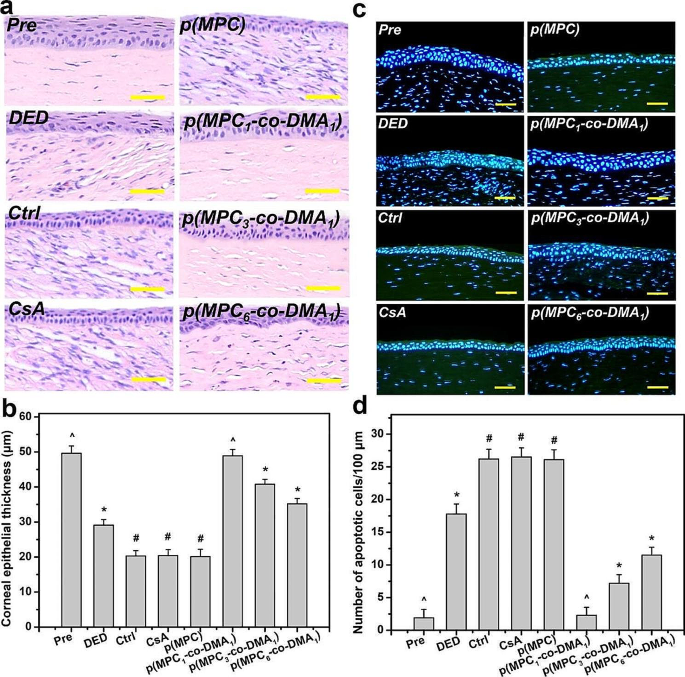
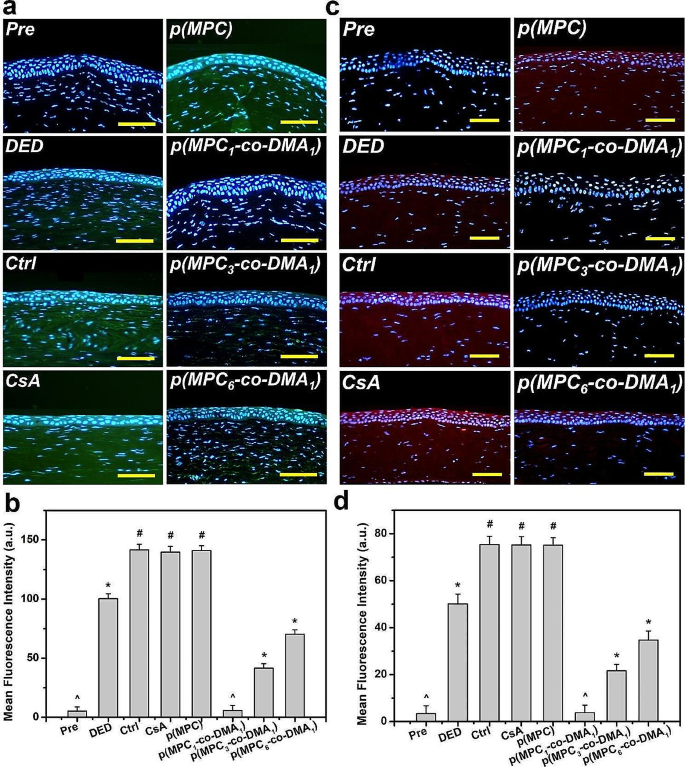
Tissue samples had been taken on the endpoint (i.e., at 4 days postoperatively) to find out the histological adjustments in response to completely different remedies. The corneal epithelia for ATS-only therapy group (Ctrl group), CsA group, and p(MPC) group had been thinner than that for the DED group (Fig. 8a). In distinction, p(MPC-co-DMA)-treated animals confirmed considerably greater tissue thicknesses (p < 0.05) amongst all teams (Fig. 8b). Curiously, the corneal epithelial integrity for the p(MPC1–co-DMA1) group was evenly matching that for the Pre group. TUNEL assays had been additional performed to investigate the genomic fragmentation and cell apoptosis in BAC-induced DED rabbits with topically administered medicines after 4 days (Fig. 8c and d). For the DED group, the apoptotic corneal epithelial cells emitted an intense inexperienced fluorescence, indicating the connection between irritation and mobile apoptosis [72]. For the CsA and p(MPC) teams, the TUNEL-labeled apoptotic cells nonetheless displayed intense inexperienced dye of DNA fragments after a 4-day therapy. This strongly implies that CsA and p(MPC) had been ineffective remedies for DED. Nonetheless, the p(MPC-co-DMA) teams exhibited profitable inhibition of cell apoptosis and there’s a vital lower (p < 0.05) within the variety of apoptotic cells and in inexperienced fluorescence after 4 days of topical administration. The smaller sign for ROS fluorescence for the p(MPC-co-DMA) teams additionally reveals that there’s a cytoprotective impact in opposition to reactive species (Fig. 9a and b). Tear movie hyper-osmolarity brought on by desiccating environmental stress can up-regulate the secretion of those pro-inflammatory cytokines (e.g. IL-6 and TNF-α), subsequently triggering continual immune-based irritation cascade and finally resulting in the disruption of corneal epithelial barrier [81]. As depicted in Fig. 9c and d and S16, the quantities of pro-inflammatory components in corneal epithelial tissue after p(MPC-co-DMA) instillation lower, in comparison with different nanoformulations. A mix of the hydration lubrication of MPC and the antioxidant motion of DMA is efficient in treating ocular dryness. Remarkably, all the indications in histological research of p(MPC1–co-DMA1)-treated group had been akin to that within the Pre group, which considerably helps the therapeutic efficacy of the polymer composition for DED. The outcomes for shielding corneal cells from a hyperosmotic atmosphere, mitigating mobile apoptosis/irritation, and suppressing ROS manufacturing present the synergistic therapeutic impact of p(MPC-co-DMA).
Histological evaluation for corneal safety and anti-apoptosis functionality. (a) Histological photos of corneal epithelium, (b) corneal epithelial thickness, (c) relative TUNEL space of the cornea and (d) TUNEL staining imply fluorescence depth of corneal epithelium in rabbit eyes at preoperation (Pre) and people with experimentally induced dry eye (DED) earlier than and after 4 days of topical administration of varied formulations. DED-induced animals receiving ATS with out polymers and drug function management group (Ctrl). Scale bars: 50 μm. Values are imply ± SD (n = 6). *p < 0.05 vs. all teams; #p < 0.05 vs. Pre, DED, p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams; ^p < 0.05 vs. DED, Ctrl, CsA, p(MPC), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams
Histological evaluation for antioxidation and anti-inflammation functionality. (a) Fluorescence photos and (b) imply fluorescence depth of corneal epithelium in rabbit eyes and people with experimentally induced DED 4 days after varied samples administration. DED animals receiving ATS with out nanomaterials and drug served as management teams (Ctrl). Inexperienced fluorescence is DCFH-DA-positive staining. Scale bars: 100 μm. (c) IL-6 immunofluorescence staining photos and (d) imply fluorescence depth of corneal epithelium in rabbit eyes and people with experimentally induced DED 4 days after varied samples administration. DED animals receiving ATS with out nanomaterials and drug served as management teams (Ctrl). Crimson fluorescence is IL-6-specific antibody staining. Scale bars: 50 μm. Values are imply ± SD (n = 6). *p < 0.05 vs. all teams; #p < 0.05 vs. Pre, DED, p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams; ^p < 0.05 vs. DED, Ctrl, CsA, p(MPC), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams
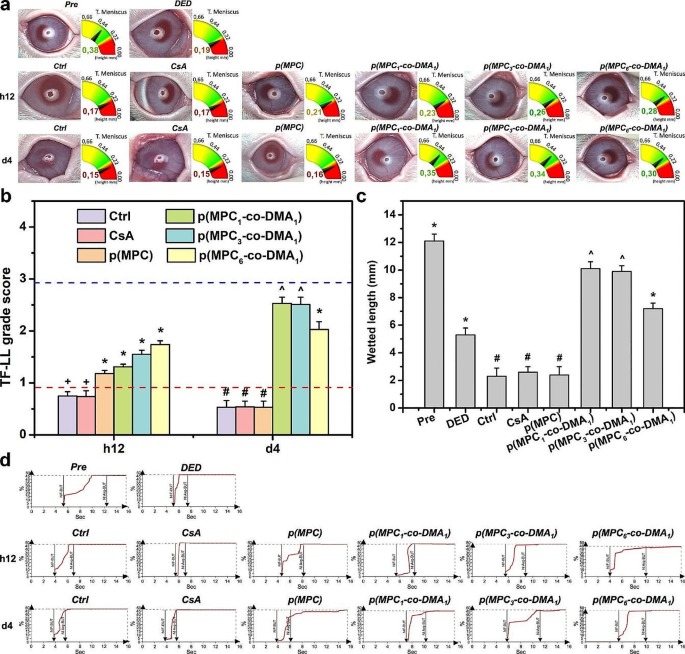
Medical evaluation of nanoformulations. (a) The pictures present tear meniscus peak (TMH) measurement, lipid layer (TF-LL) sample and (b) grading scale of interferometric patterns of Pre, DED, Ctrl, CsA, p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams at h12 and 4 days. The blue and crimson sprint line represents the worth of Pre and DED group, respectively. (c) The wetted size of the Schirmer paper strip for the rabbit eyes earlier than drug administration (Pre) and people with experimentally induced DED 4 days after topical administration of CsA, p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1). DED animals receiving synthetic tear answer (ATS) with out nanomaterial and drug function management teams (Ctrl). Values are imply ± SD (n = 6). *p < 0.05 vs. all teams; +p < 0.05 vs. Pre, DED, p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1); #p < 0.05 vs. Pre, DED, p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1); ^p < 0.05 vs. Pre, DED, Ctrl, CsA, p(MPC), and p(MPC6–co-DMA1). (d) The tear movie first break-up time (NIF-BUT) and tear movie common break-up time (NIAvg-BUT) measurements of Pre, DED, Ctrl, CsA, p(MPC), p(MPC1–co-DMA1), p(MPC3–co-DMA1), and p(MPC6–co-DMA1) teams at h12 and 4 days
The peak of the tear meniscus and the lipid layer thickness for the tear movie had been measured utilizing an ocular floor analyzer. The conventional vary for tear meniscus peak is from 0.2 to 0.5 mm. For the DED group, the tear meniscus peak in is lower than 0.2 mm [82, 83]. As depicted in Fig. 10a, all polymer samples show a rise in tear quantity after a h12 topical therapy. Nagai and colleagues confirmed that p(MPC) can improve corneal water retention, improve tear quantity, and lengthen tear movie break-up time with out growing the entire mucin content material within the tear movie of a traditional rabbit [25]. The tear meniscus peak of p(MPC) and different copolymer teams had been ≥ 0.21 mm (extra distinguished than Ctrl and CsA teams at 0.17 mm) at h12 postoperatively. p(MPC) is non-fouling however the zwitterionic polymer alleviates dry eye signs due to its lubricity, and dietary supplements the meibomian lipid layer in precorneal tear movie. Subsequently, a rise within the tear movie lipid layer (TF-LL) thickness helps this assumption (Fig. 10b). Nonetheless, solely catechol-functionalized polymers can prolong the pharmacological impact after a 4-day therapy owing to their superior mucoadhesive and bioactive properties of DMA (Fig. 10c). All p(MPC-co-DMA) teams confirmed thickness ≥ 0.3 mm within the tear meniscus layer and had been approaching the TF-LL rating of three (Fig. 10a and b), suggesting the tear movies had been within the strategy of regeneration.
The tear movie break-up time (BUT) is an index for direct medical examination of the tear movie, which refers back to the time from the primary full blink to the looks of the primary dry spot on the tear movie. The BUT index is brief whether it is lower than 10 s, as a result of there’s inadequate tear secretion or an abnormality within the ocular floor. In medical phrases, the typical rupture time for regular people is about 10 ∼ 13 s, however the common rupture time for DED sufferers is about 7 ∼ 8 s. After a h12 therapy, all formulations of MPC-containing polymer improve the typical break-up time of tear movie, indicating stabilization of tear osmolarity (Fig. 10d and Desk S4). Remarkably, the interblink interval of rabbits prolonged considerably in all p(MPC-co-DMA) teams at 4 days postoperatively. This knowledge confirms that catechol-functionalized polyzwitterion has a superior synergistic therapeutic impact, together with hydration lubrication, supplementing of the lipid layer, mucoadhesion and anti-oxidation/anti-inflammation exercise.